A Guide to the Geology of the Sinnett-Thorn Mountain Cave System, Pendleton County, West Virginia
CHRISTOPHER S. SWEZEY1,5,6, NADINE M. PIATAK2, LORA A. CHIEHOWSKY1, R. LEE HADDEN3,5, PAUL C. HACKLEY1, COLIN A. DOOLAN1, WILMA B. ALEMAN GONZALEZ4, PATRICIA A. BINGHAM5,6, and ROBERT B. HOKE5,6
1 U. S. Geological Survey, Mailstop 956 National Center, Reston, Virginia 20191 USA
2 U. S. Geological Survey, Mailstop 954 National Center, Reston, Virginia 20191 USA
3 U. S. Geological Survey, Mailstop 950 National Center, Reston, Virginia 20191 USA
4 U. S. Geological Survey, Mailstop 926A National Center, Reston, Virginia 20191 USA
5 National Speleological Society (NSS)
6 Potomac Speleological Club (PSC)
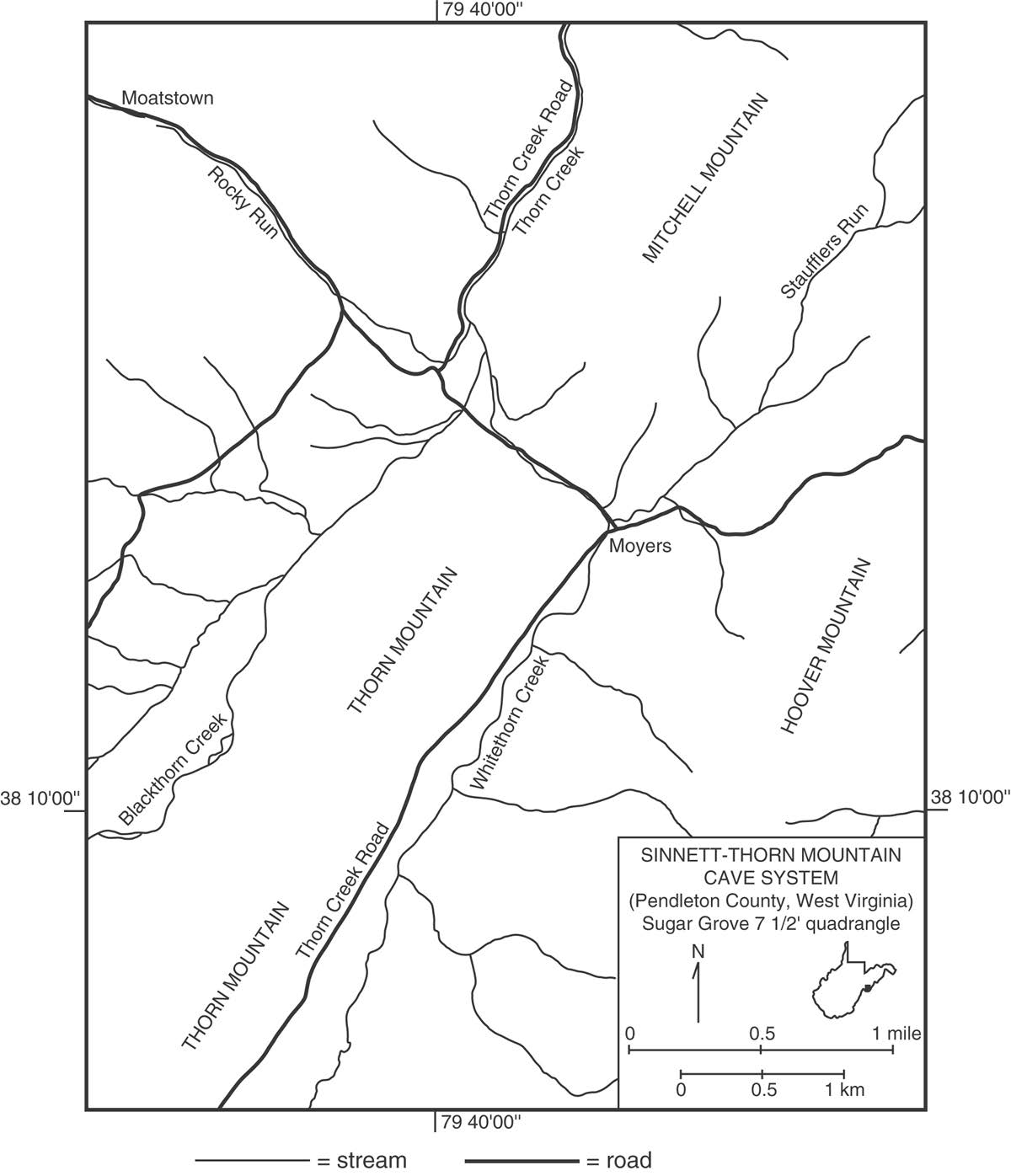
The Sinnett-Thorn Mountain Cave System is located on private property west of the town of Moyers in Pendleton County, West Virginia (Fig. 1). The cave system is comprised of Sinnett Cave (near the base of Thorn Mountain) and Thorn Mountain Cave (near the top of Thorn Mountain). Although Sinnett Cave and Thorn Mountain Cave were once thought to be separate, a small connection between the two caves was established in 1962 (Giles, 1962). Today, the entrances to both Sinnett Cave and Thorn Mountain Cave are guarded by locked metal gates (“bat gates”), and access to the caves may be obtained through the Potomac Speleological Club (see the following website for information about access: psccaving.com). Additional information about caves and caving may be obtained from the National Speleological Society (http://www.caves.org).
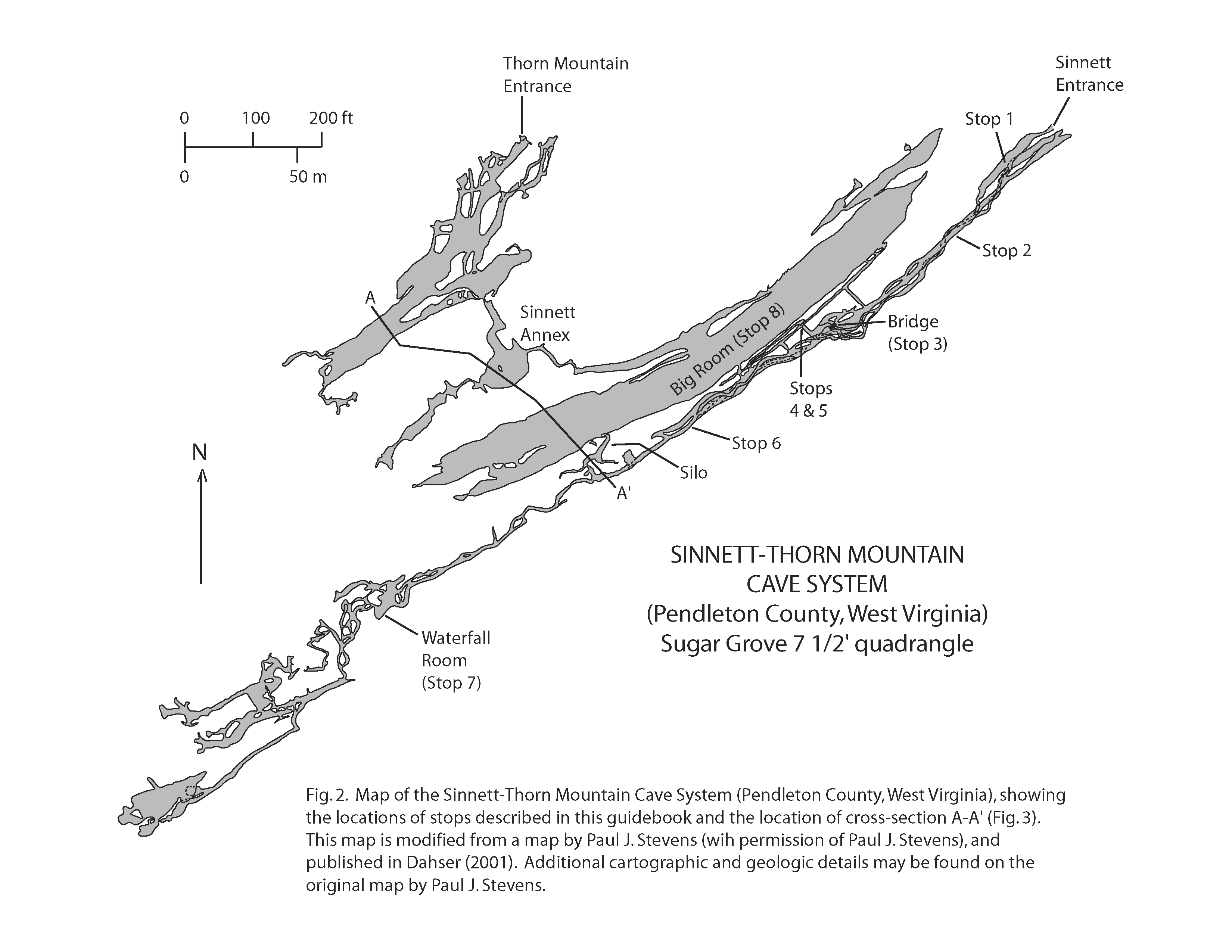
This guide provides a general introduction to the geology of the Sinnett-Thorn Mountain Cave System (Figs. 2 and 3). The guide is organized for a trip going from the Sinnett Cave entrance to the Waterfall Room and then ending in the Big Room at the top of the Silo (Fig. 2). Along the trip route, there are specific stops where various topics are discussed (cave meteorology, speleothems, cave soils, faults, cave passage morphology, mineralogy, hydrology, stratigraphy, and fossils). All of the stops are located within the Sinnett part of the cave system, but the text and figures contain additional information pertaining to the Thorn Mountain part of the cave system.
Stop One: Cave Meteorology
(JUST INSIDE THE ENTRANCE TO SINNETT CAVE)
The topic at this first stop is cave meteorology (much of the following general information on cave meteorology is from Wigley and Brown, 1976). Important subjects of cave meteorology include (1) air flow, (2) temperature, and (3) humidity. Before reading the remainder of the text below, take a minute to think about how the air flow, temperature, and humidity at Stop 1 differ from the conditions just outside the cave.
In most caves, there is some air flow. This air flow may be caused by changes in air temperature, pressure, and (or) density outside the cave. Air flow may also be caused by the resonance of air in large cave chambers, by the entrainment of air by flowing water, and by changes in the volume of air in a cave when the cave is flooded.
For the Sinnett-Thorn Mountain Cave System, which has two entrances at different elevations, temperature and pressure differences inside and outside the cave create an air flow pattern that is referred to as a “chimney effect.” A temperature difference will produce a pressure difference between the cave entrances, and air will flow either into or out of the cave in order to equilibrate the temperature and pressure differences between the entrances. When the air inside the cave is warmer than the air outside the cave, air will flow into the lower cave entrance (at Stop 1) and out of the upper cave entrance. In contrast, when the air inside the cave is colder than the air outside the cave, air will flow into the upper cave entrance and out of the lower entrance of the cave (at Stop 1).
Changes in the direction of air flow into and out of a cave have been referred to as the “breathing” of the cave (Faust, 1947; Anonymous, 1959; Plummer, 1962, 1969; Wigley, 1967). Long-period breathing is caused by periodic changes in atmospheric temperature and pressure outside of the cave. In contrast, short-period breathing may be caused by a resonance phenomenon, similar to that which is produced when one blows over a bottle. The resonance frequency of a bottle is high, and the resulting oscillations are observed as sound waves. In contrast, the resonance frequency of a cave is low, and the resulting oscillations are observed as periodic reversals in the direction of air flow at the mouth of a large cave chamber. Breathing of Sinnett Cave was postulated by Anonymous (1959) and later confirmed by Plummer (1969), who documented two resonance frequencies of “breathing.” One frequency of 28.9 mHz [= 28.9 millihertz or 0.0289 cycles per second; in other words, 1 “breathing” cycle = approximately 35 seconds] occurs because the Big Room (Fig. 2) and its entrance passage act as the volume and neck of a simple resonator. A second frequency of 90.2 mHz [1 “breathing” cycle = approximately 11 seconds] occurs from the resonance of the Sinnett Annex (Fig. 2) and the passage connecting the Sinnett Annex to the Big Room.
In addition to temperature, pressure, and resonance phenomena, some air flow in Sinnett Cave may be caused by the stream pulling air out of the cave along with the flowing water. This process should result in a return air flow along the cave ceiling or through other passages, but it may be difficult to distinguish this particular phenomenon from other air flow processes. In a related situation, rapidly rising water in a cave that is flooding may displace air at a rate that is fast enough to produce an outward flowing current of air (Myers, 1962). Such air flow caused by flooding might occur in the lower and western part of Sinnett Cave, where there is more running water.
Cave air flow influences both cave temperature and cave humidity. Although caves are often perceived as having relatively constant temperature and humidity, caves do experience changes in these parameters. In general, the greater the air exchange between the cave and the outside, the greater will be the temperature and humidity changes in the cave. With increasing distance from the cave entrance, the magnitudes of temperature and humidity fluctuations decrease.
As air flows into a cave, the air is either cooled or warmed by contact with the cave walls. At the same time, moisture may either evaporate from the cave walls or condense onto the cave walls, depending upon whether the walls are warmer or cooler than the dew point temperature of the air (the “dew point temperature” is the temperature to which a sample of air must be cooled to make it saturated with moisture). Thus, there is direct heat transfer between the air and the cave walls, and there is latent heat transfer via moisture evaporation and condensation.
On February 1, 2003, we took several measurements of temperature and relative humidity in Sinnett Cave, using ACU>RITE thermometer and humidity instruments (purchased for about $2 each at K-Mart). The temperature measurements were “dry bulb” measurements of the air temperature, and the relative humidity measurements indicated how close the air was to water saturation (with a relative humidity of 100 % being equivalent to complete water saturation). Our measurements were as follows:
| Location (see Fig. 2) | Temperature (degrees F) | Relative Humidity (%) | Time of Reading (& equilabration time) |
|---|---|---|---|
| Stop 4 (Fault) | 44 | 88 | 18:35 (250 minutes) |
| Base of Silo | 51 | 63 | 14:24 (5 minutes) |
| Base of Silo | 50 | 66 | 1427 (8 minutes) |
| Waterfall Room | 54 | 79 | 15:00 (5 minutes) |
| Waterfall Room | 54 | 82 | 15:15 (20 minutes) |
| Big Room | 60 | 75 | 17:02 (30 minutes) |
The readings at Stop 4 and in the Big Room may be more reliable because the instruments were left out for greater amounts of time at these locations and the instruments were not influenced by the presence of ten warm cavers in the area.
Stop Two: Speleothems
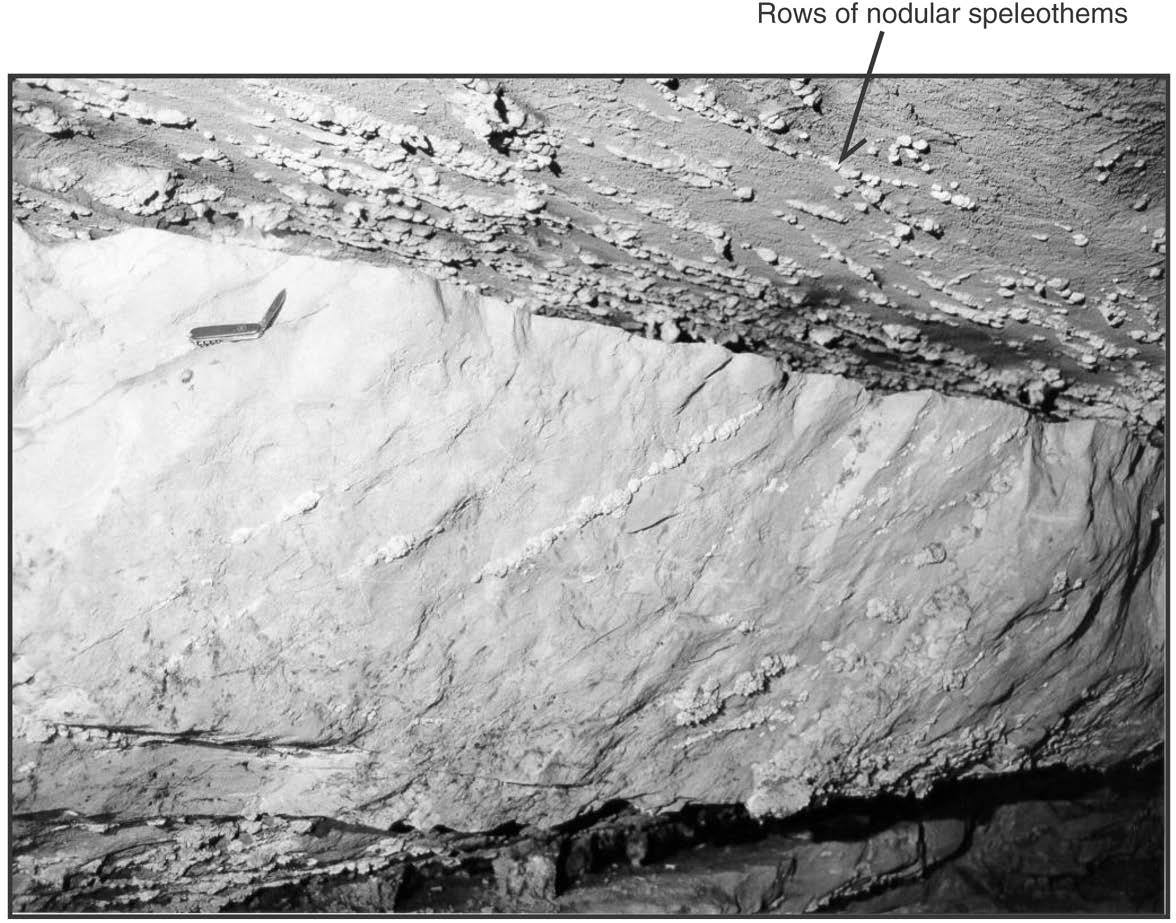
At this stop, rows of cauliflower-shaped nodules (“coralloids”), 1-2 cm in diameter, are present on the ceiling and walls of the cave along the south side of the passage (Fig. 4). These nodules are white deposits of calcite, which is a calcium carbonate (CaCO3) mineral. Most of these nodules are arranged along lines because they have formed along linear fractures. Groundwater seeps through these fractures, and calcium carbonate that is dissolved in the groundwater precipitates when the groundwater reaches the ceiling and (or) walls of the cave.
Mineral deposits that form within caves are called speleothems, and they may be classified according to their shape. Common speleothems include stalactites, stalagmites, columns, helictites, and coralloids (e.g., Hill and Forti, 1997). Stalactites extend down from the cave ceiling, stalagmites extend up from the cave floor, and columns are speleothems that span the entire distance from the cave ceiling to the cave floor. Helictites are contorted speleothems that twist in various directions, and “coralloid” is a general term for a nodular or globular speleothem.
Speleothems are not very abundant in the Sinnett part of the cave system, although some columns are present between Stop 2 and Stop 3. In addition, various stalactites and stalagmites are present in the western end of the cave, where there is more water (Ferguson, 1964). In the Thorn Mountain part of the cave system, however, many small speleothems are present in the form of calcite crystals that extend horizontally from the cave walls. These crystals are 1-5 cm long, and some have secondary needle-shaped crystals that extend outward from the main crystal surface. At some locations, these crystals terminate with spherical forms (such crystals are called “globularites”). According to studies by Broughton (1969, 1972) and Broughton and Murray (1971), the spherical tips of the Thorn Mountain Cave globularites are about 6 mm in diameter, whereas the main crystals are 1-5 cm in length and up to 5 mm in width. The spheres are solid and they are composed of concentric layers of radiating, micro-faceted fibers of calcite. Many of the concentric layers exhibit different colors (brown, yellow, cream, and white) caused by various impurities (such as iron and manganese) within the calcite. Although originally reported as gypsum, it was later determined that these spheres are composed of calcite.
Stop Three: Cave Soil and “Saltpeter”
At this stop, an old wooden plank forms a bridge across a small chasm (Fig. 5). If you follow the bridge to the northeast (Fig. 2), you will enter a narrow fissure that extends up about 38 m (125 ft) to the Big Room. Yellow-orange dirt is abundant on the cave floor at the base of the fissure. This dirt is cave soil that contains nitrate, which was used in making gunpowder before the 1870s.
Many of the caves in Pendleton County were mined for nitrate for the purpose of making gunpowder (Faust, 1964; Powers, 1981; Ellen and Garton, 2001; Taylor, 2001). These caves were part of the “Old Salt Peter Route,” an extensive series of caves that were mined for nitrate in Virginia and West Virginia (Faust, 1964). During the American Civil War, Pendleton County remained loyal to the Confederacy, and there are various accounts of Union soldiers and (or) guerrilla forces destroying Confederate buildings and nitrate leaching vats in the county and elsewhere along the “Old Saltpeter Route” (Faust, 1964; Smith, 1995; Ellen and Garton, 2001).
For Sinnett Cave, accounts of the nitrate mining and refining process are given by Faust (1956, 1964) and Davies (1965). According to these authors, cave soil was dug out of the Big Room (Stop 7 of this guide) and then dumped down the pits and fissures to the main passage of Sinnett Cave. At Stop 3 (near the wooden bridge), the soil was placed in bags and carried out of the cave. From the cave, the soil was carried across Thorn Creek and placed in leaching vats with fresh water, which leached calcium nitrate from the soil. After standing for several days, this water (called the “mother liquor”) was drained from the vats and into a collecting trough. In a separate container, wood ashes (rich in potassium hydroxide) were leached with water, and this wood ash leachate was combined with the cave soil leachate (rich in calcium nitrate). After several additional steps of concentration and purification (for details, see Wigginton, 1979), crystals of potassium nitrate (called “salt peter” or “saltpeter”) were recovered from the liquid. This potassium nitrate was then mixed with charcoal and sulfur to produce gunpowder.
Preliminary analyses indicate that concentrations of nitrate in the soil of Sinnett Cave are generally between 400 and 600 parts per million (Hadden et al., 2003-in press). This information is based on the analyses of three soil samples from the Big Room (collected on March 29, 2003, with written permission of the cave owner). The three samples were sent to an agricultural soil testing laboratory for analysis, and the results of the analyses were as follows:
| USGS Sample Number | Nitrate Concentration (in parts per million) | Nitrate Concentration (in weight percent) |
|---|---|---|
| SNT1-PB | 482 | 0.05 |
| SNT2-PB | 478 | 0.05 |
| SNT3-PB | 580 | 0.06 |
These nitrate concentrations in the soil of Sinnett Cave are similar to nitrate concentrations in other saltpeter caves. For example, three soil samples from Organ Cave (collected on September 22, 2003, with written permission of the cave owner) in Greenbrier County (West Virginia) were also sent to the same agricultural soil testing laboratory for analysis, and the results of these analyses were 362, 820, and 1014 ppm. In a separate study, reported in Hill (1981), the analysis of five soil samples from Dixon Cave (Kentucky) revealed nitrate concentrations of 483, 953, 1103, 2313, and 2955 ppm. Hill (1981) also reported on the analysis of seven soil samples from Mammoth Cave (Kentucky), with nitrate concentrations being 1108, 1794, 9126, 12059, 12865, 15660, and 21734 ppm.
Stop Four: Fault
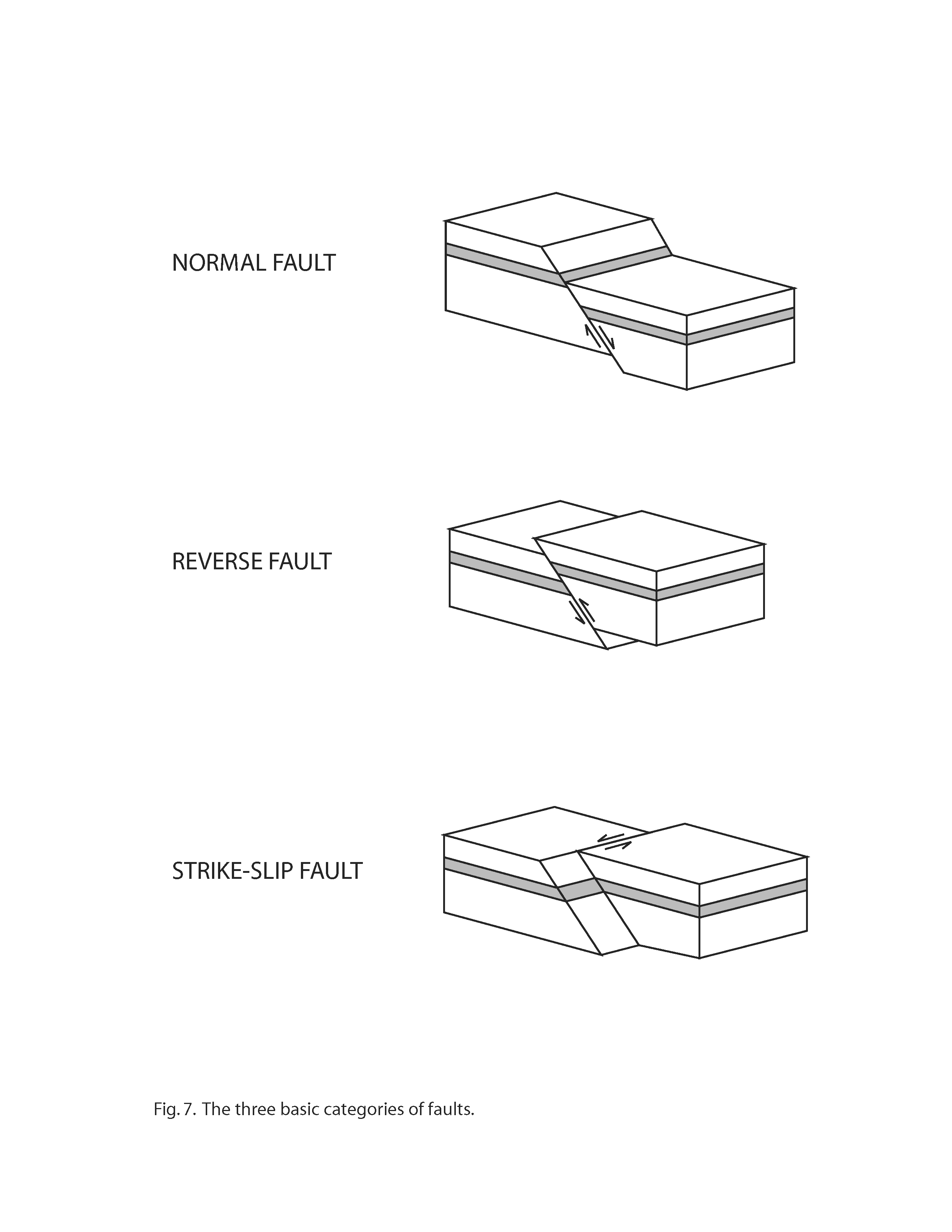
At this stop, a fault is visible on the western side of the cave passage (Fig. 6). A fault is a fracture in rock where one side of the fracture has moved relative to the other side. Geologists classify faults into the following three basic categories (Fig. 7), based on the direction of movement along the fault plane: (1) normal faults, (2) reverse faults, and (3) strike-slip faults. A normal fault is one in which the rocks above the fault plane move down relative to the rocks below the fault plane. A reverse fault is one in which the rocks above the fault plane move up relative to the rocks below the fault plane. A strike-slip fault is one in which movement along the fault plane is horizontal rather than vertical. The fault at Stop 4 is a reverse fault. Reverse faults develop as a result of compressive forces (for example, the compression that created the Appalachian Mountains).
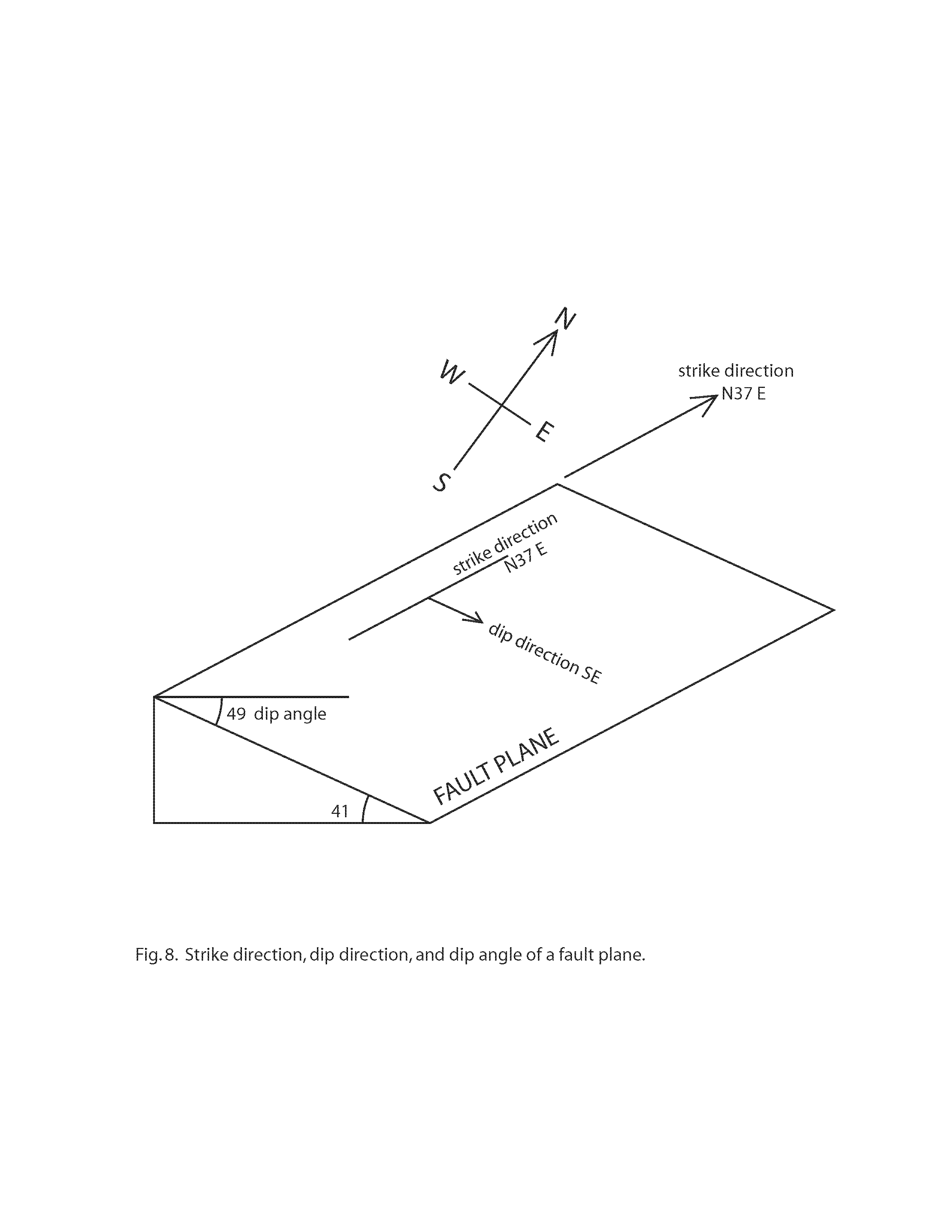
When describing a particular fault, geologists measure the “strike” and “dip” of the fault plane (Fig. 8). The dip is the angle of inclination (in the direction of steepest descent) of the fault plane, as measured from a horizontal line on that plane. The strike is the direction of the line formed by the intersection of the fault plane with a horizontal plane, and this intersection is at right angles to the dip direction. For the fault at Stop 4, the strike direction is N37 degrees E, the dip direction is SE (N143 degrees East), and the dip angle is 49 degrees. The strike direction of N37 degrees E is approximately parallel to the overall trend of Thorn Mountain (see Fig. 1).
Stop Five: Passage Morphology
In the Sinnett-Thorn Mountain Cave System, many of the ceilings and floors of the cave passages have developed parallel to the limestone beds. For Stop 5, look down the passage extending southwest from Stop 4, and note how the passage has a wedge-shaped outline (Fig. 9). The ceiling of the passage is parallel to the steeply dipping beds on the northwest side of the fault. Passages with wedge-shaped outlines are common on the northwest side of the fault, where the cave floors and ceilings follow limestone beds that dip steeply to the southeast (i.e., Sinnett Big Room, Sinnett Annex, and Thorn Mountain Cave). In contrast, on the southeast side of the fault, the beds are approximately horizontal and the cave passages have a more rectangular outline (e.g., Stops 2 and 3). Look at Fig. 3 and compare the outline of the Big Room (where the beds dip steeply to the southeast) with the outline of the main Sinnett Cave passage (where the beds are approximately horizontal).
Stop Six: Gypsum
At this stop, white speleothems composed of gypsum (gypsum flowers and gypsum needles) are present along thin beds in the limestone (Fig. 10). Gypsum is a hydrated calcium sulfate mineral (CaSO4*2H2O). For most cave gypsum, the calcium (Ca) usually comes from the surrounding limestone, but the source of sulfur (S) may be more difficult to determine. Possible sources of sulfur include other gypsum deposits, sulfide minerals (such as pyrite), and material containing organic compounds (such as petroleum or coal containing organically bound sulfur).
The analysis of sulfur isotopes is one means of identifying potential sources of the sulfur (Faure, 1986). Isotopes are atoms of the same element that have the same number of protons and electrons, but different numbers of neutrons. All isotopes of sulfur have 16 protons and 16 electrons, but the number of neutrons can range from 16 to 20. The different isotopes of sulfur are denoted according to the number of protons plus the number of neutrons. For example, 32S is an isotope of sulfur consisting of 16 protons and 16 neutrons [16+16=32], whereas 34S is an isotope of sulfur consisting of 16 protons plus 18 neutrons [16+18=34]. According to convention, the isotopic composition of any element is expressed as enrichment or depletion relative to a standard of known isotopic composition. For sulfur, this standard is a specific meteorite (the Canyon Diablo troilite) that was found in Meteor Crater, Arizona.
The isotopic composition of sulfur of a given gypsum sample is reported as d34S, which is the difference in parts per thousand (per mil) between the 34S/32S ratio of a sample and the 34S/32S ratio of a standard. Typically, d34S is reported according to the following equation:
d34S = {[(34S/32S)sample – (34S/32S)standard] / (34S/32S)standard} * 1000If d34S is a positive value, then the sample contains more 34S than does the standard (i.e., the reference meteorite). If d34S is negative, then the sample contains less 34S than does the standard. For example, if a sample has a d34S of –5.0 parts per thousand, then there are 5 parts per thousand (or 0.5%) less 34S in the sample than in the standard.
The analysis of four samples of gypsum collected from the Sinnett part of the cave system (collected by the senior author, with written permission of the cave owner) revealed the following isotopic values of sulfur:
| USGS Sample Number | Isotopic Composition (d34S in parts per thousand) | Gypsum Form (Morphology) |
|---|---|---|
| SNT-2-CS | -7.5 | gypsum flowers |
| SNT-3-CS | -10/7 | gypsum flowers |
| SNT-4-CS | -8.1 | gypsum crust |
| SNT-5-CS | -9.2 | gypsum crust |
These negative isotopic compositions of sulfur in cave gypsum are common when the sulfur is derived from sulfide minerals, such as pyrite in the host limestones and (or) nearby shales (Swezey et al., 2002; Swezey and Piatak, 2003-in press). For the formation of gypsum in Sinnett Cave, groundwater in nearby strata probably oxidized sulfide minerals and formed sulfates, which were released into the water. Calcium ions were also released into this groundwater as the groundwater reacted with the surrounding limestone. Gypsum then formed (either by evaporation or by a chemical reaction) when this sulfate-bearing and calcium-bearing groundwater in the limestone came into contact with the cave atmosphere.
Stop Seven: Hydrology (Waterfall Room)
At this stop, water flows down a 6 m (20 ft.) high waterfall and into a small pool (Fig. 11). From this pool, water flows east as a shallow stream on the cave floor, eventually diving beneath the Sinnett Cave entrance passage and resurging in Thorn Creek (Fig. 1) outside the cave at the base of the slope beneath the cave entrance. At certain times of the year, locations where this cave water resurges in the stream outside the cave can be identified by differences in stream vegetation (the vegetation responds to temperature differences between the cave stream water and the stream water outside the cave).
Caves may contain water of different chemical compositions. Groundwater, for example, seeps through the rock, is in contact with the rock for relatively long periods of time, and contains minerals dissolved from the surrounding rock. In contrast, surface water and subsurface stream water typically flow more rapidly through a landscape and may not contain as many minerals dissolved from the surrounding rock. At Stop 7, both seepage groundwater and subsurface stream water are present. The seepage groundwater contains dissolved minerals and has formed speleothems, whereas the subsurface stream water (e.g., the waterfall and stream) does not contain as many dissolved minerals and has not formed speleothems. Analyses of a water sample taken from the pool at Stop 7 revealed that the concentration of sulfate of this water was less than 2 milligrams per liter (the detection limit of the analytical equipment), suggesting that the sulfur in the gypsum speleothems at Stop 6 is probably transported by seepage groundwater rather than by subsurface stream water.
Although the subsurface stream water is not forming speleothems, this water has influenced cave development. Notice that the rock around the waterfall has been eroded and (or) dissolved so as to form a cylindrical pit. This cylindrical morphology is often associated with cave waterfalls, and may be used to identify the locations of former waterfalls (or waterfalls that are only active periodically). As you leave Stop 7 and go back towards the Silo (Fig. 2), note the presence of several cylindrical pits in the cave ceiling.
Stop Eight: Stratigraphy and Fossils
[“The Big Room;” also known as “The Long Room” and “The Hall of the Mountain King”]
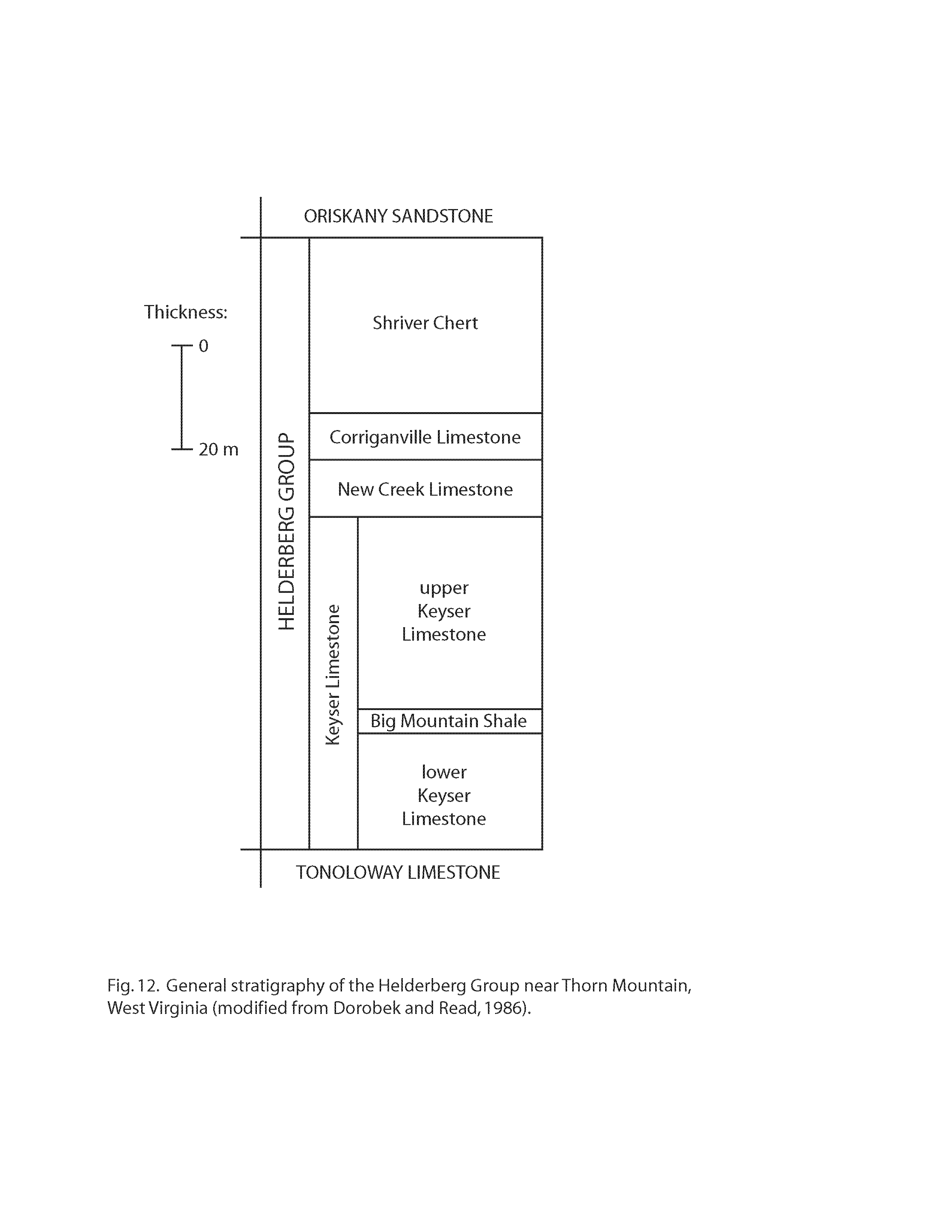
At this stop, it is possible to identify several fossils and different stratigraphic units (i.e., different layers of rock in which the cave has developed). The Sinnett-Thorn Mountain Cave System has developed in carbonate rocks (rocks containing CO32-) that geologists call the Helderberg Group. These rocks are of Late Silurian to Early Devonian age (approximately 408-390 million years old), and they are approximately 120 m thick in the vicinity of Thorn Mountain (Dorobek and Read, 1986). The Helderberg Group, in turn, is divided (from base to top) into the following four units (Fig. 12): (1) the Keyser Limestone (including the lower Keyser Limestone, the Big Mountain Shale, and the upper Keyser Limestone); (2) the New Creek Limestone; (3) the Corriganville Limestone; (4) the Shriver Chert. The Sinnett-Thorn Mountain Cave System has developed in the Keyser, New Creek, and Corriganville Limestones (Fig. 3; see also Stokowski, 1979).
The Keyser Limestone, which is the lowermost formation of the Helderberg Group, consists primarily of massive limestone, nodular-bedded limestone, shaly limestone, and crossbedded to horizontally bedded limestone. The type section is at a quarry near the town of Keyser in Mineral County, West Virginia (Ulrich, 1911; Swartz, 1913). Near the town of Moyers (Fig. 1), the total thickness of the Keyser Limestone is about 80 m (Swartz, 1929).
The New Creek Limestone, which overlies the Keyser Limestone, is a crinoid grainstone to packstone (classification of Dunham, 1962). In some publications, this limestone has been called the Coeymans Limestone (e.g., Ulrich, 1911; Swartz, 1929), on account of similarities with the Coeymans Limestone in New York. However, this limestone in West Virginia cannot be traced through the intervening areas between West Virginia and New York. As a result, the name Coeymans Limestone has been discontinued in areas south of central Pennsylvania, and the name New Creek Limestone is used instead (Bowen, 1967). The type section of the New Creek Limestone is at a quarry on the north side of US Route 50, 0.5 miles (0.8 km) south of the town of New Creek in Mineral County, West Virginia. This quarry is 100 yards (91 m) east of a stream called New Creek where US Route 50 crosses New Creek Mountain (Bowen, 1967). Near the town of Moyers (Fig. 1), the total thickness of the New Creek Limestone is about 6 m (Swartz, 1929).
The Corriganville Limestone, which overlies the New Creek Limestone, is a brachiopod packstone to grainstone with occasional chert nodules, horn corals, and gastropods. In some publications, this limestone has been called the New Scotland Limestone (e.g., Ulrich, 1911; Swartz, 1929), on account of similarities with the New Scotland Limestone in New York. However, this limestone in West Virginia cannot be traced through the intervening areas between West Virginia and New York. As a result, the name New Scotland Limestone has been discontinued in areas south of central Pennsylvania, and the name Corriganville Limestone is used instead (Head, 1972). The type section of the Corriganville Limestone is at a railroad cut 0.3 miles (0.5 km) southeast of the town of Corriganville in Allegheny County, Pennsylvania (Head, 1972). Near the town of Moyers (Fig. 1), the total thickness of the Corriganville Limestone is about 7 m (Swartz, 1929).
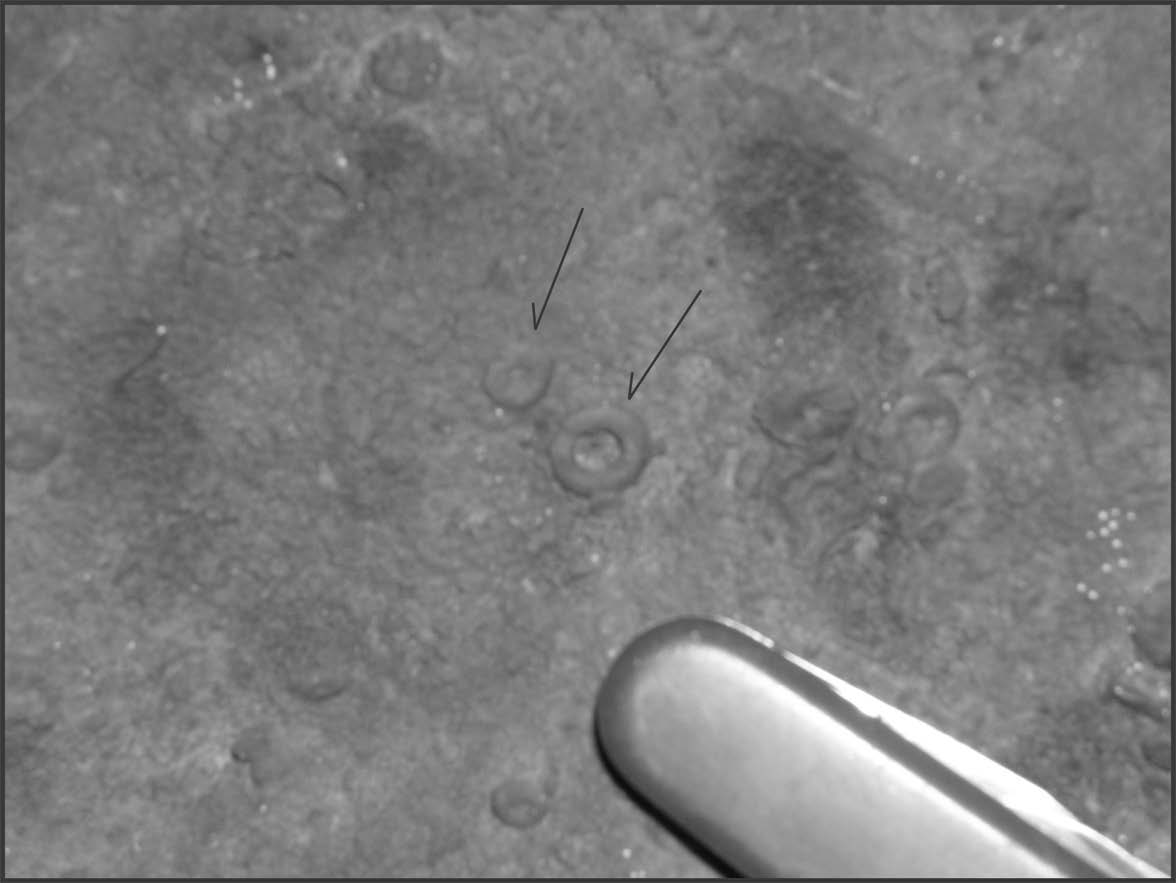
The New Creek Limestone and the overlying Corriganville Limestone contain characteristic fossils, which can be useful for identifying these units (e.g., Lawrence and Melson, 1960; DeWall, 1968; Dorobek and Read, 1986). In many places, the New Creek Limestone contains abundant fragments of fossil crinoids, marine animals (sometimes called “sea lilies”) that have “stems” comprised of disks that are usually 0.5-2 cm in diameter (Fig. 13). The New Creek Limestone has been interpreted by Dorobek and Read (1986) as a marine deposit of carbonate debris (mostly pieces of crinoid stems) reworked in shallow water (< 15 m deep) by waves and tides. In contrast, the overlying Corriganville Limestone contains abundant fossil shells (called brachiopods; Fig. 14) and occasional corals and gastropods. Dorobek and Read (1986) interpreted the Corriganville Limestone as a marine deposit that developed in water depths of 15-25 m. Thus, the progression from the New Creek Limestone to the overlying Corriganville Limestone represents a change from relatively shallow water to relatively deeper water.
Many of the caves in Pendleton County have developed along the contact between the New Creek Limestone and the Corriganville Limestone. This contact is well exposed on the south wall of the Big Room about 12 m (40 ft) east of the Silo (Fig. 2), and it is also well exposed along the passage leading out of the Big Room towards the connection with the Sinnett Annex (Fig. 2). Along this passage, the New Creek Limestone is visible along the north wall (note abundant crinoid stems), whereas the overlying Corriganville Limestone is visible along the ceiling and south wall (note abundant brachiopod shells). Chert nodules mark the top of the New Creek Limestone in some places along this passage.
Stop 8 is the last stop of the guide. We hope that you have enjoyed the tour of Sinnett Cave. This tour has presented information on the topics of cave meteorology, speleothems, cave soils, faults, cave passage morphology, mineralology, hydrology, stratigraphy, and fossils. There are many other topics associated with the geology of caves, however, and additional information may be obtained from the National Speleological Society (http://www.caves.org) and from the U. S. Geological Survey (http://www.usgs.gov).
Acknowledgements
Thanks to the Potomac Speleological Club (PSC), Charlotte Hoover, the staff at Thompson’s Restaurant (Franklin, WV), and everyone who participated in the various cave trips associated with this project. Thanks also to Robert R. Seal II and Gregory A. Wandless for aid in sulfur isotope analysis. Reviews by USGS geologists John Repetski and David Weary improved this manuscript.
References
- Anonymous, 1959, The “Ins” and “Outs” of Breathing Cave: National Speleological Society (NSS) News, v. 17, p. 6-8.
- Bowen, Z.P., 1967, Brachiopoda of the Keyser Limestone (Silurian – Devonian) of Maryland and Adjacent Areas: Geological Society of America Memoir 102, 103p.
- Broughton, P., 1969, Science photo of the month: National Speleological Society (NSS) News, v. 27 (3), p. 51.
- Broughton, P.L., 1972, A new calcite structure from Thorn Mountain Cave, West Virginia: Caves and Karst, v. 14 (1), p. 1-3.
- Broughton, P.L., & Murray, J.W., 1971, Globularite crystallization in Thorn Mountain Cave, West Virginia: National Speleological Society (NSS) Bulletin, v. 33 (4), p. 142.
- Dasher, G.R., ed., 2001, The Caves and Karst of Pendleton County: West Virginia Speleological Society (WVSS) Bulletin 15, 404p.
- Davies, W.E., 1965, Caverns of West Virginia (second edition): West Virginia Geological and Economic Survey, v. 19A, 330p.
- DeWall, A., 1968, The geology of the Sinnit-Thorn Mountain Cave System: The Colonial Caver, v. 2 (2), p. 10-11.
- Dorobek, S.L., & Read, J.F., 1986, Sedimentology and basin evolution of the Siluro-Devonian Helderberg Group, central Appalachians: Journal of Sedimentary Petrology, v. 56, p. 601-613.
- Dunham, R.J., 1962, Classification of carbonate rocks according to depositional texture, in: Classification of Carbonate Rocks (W. E. Ham, ed.): American Association of Petroleum Geologists (AAPG) Memoir 1, p. 108-121.
- Ellen, M., & Garton, R., 2001, Pendleton County’s saltpeter caves and their role in the Civil War, in: The Caves and Karst of Pendleton County (G. Dasher, ed.): West Virginia Speleological Survey (WVSS) Bulletin 15, p. 70-73.
- Faure, G., 1986, Principles of Isotope Geology (second edition): John Wiley and Sons, New York, 589p.
- Faust, B.S., 1947, An unusual phenomenon: National Speleological Society (NSS) Bulletin, v. 9, p. 52-54.
- Faust, B., 1956, A description of Sinnet Cave and its salt petre works: National Speleological Society (NSS) News, v. 14 (7), p. 68-70.
- Faust, B., 1964, Saltpetre caves and Virginia history, in: Caves of Virginia (H. H. Douglas, ed.): Virginia Cave Survey, Falls Church, Virginia, p. 31-55.
- Ferguson, L., 1964, Flash! — Passage beyond the waterfall rediscovered!: The Cavalier Caver, v. 6, n. 1, p. 25.
- Giles, H.N., 1962, Sinnett-Thorn Mountain Cave System (Pendleton County, West Virginia): The Cavalier Caver, v. 4 (3), p. 11-12.
- Hadden, R. L., Swezey, C. S., Piatak, N. M., & Bingham, P. A., 2003 – in press, Saltpeter mining in Sinnett Cave (Pendleton County, West Virginia) during the American Civil War: Geological Society of America, Abstracts with Programs.
- Head, J.W., 1972, Upper Silurian – Lower Devonian stratigraphy and nomenclature in the central Appalachians, in: Stratigraphy, Sedimentology, and Structure of Silurian and Devonian Rocks along the Allegheny Front in Bedford County, Pennsylvania, Allegany County, Maryland, and Mineral and Grant Counties, West Virginia (J. M. Dennison, W. de Wittt, Jr., K. O. Hasson, D. M. Hoskins, and J. W. Head, III, field trip leaders): Guidebook for the 37th Annual Field Conference of Pennsylvania Geologists, p. 96-103.
- Hill, C.A., 1981, Origin of saltpeter: National Speleological Society (NSS) Bulletin, v. 43, p. 110-126.
- Hill, C., & Forti, P., 1997, Cave Minerals of the World [second edition]: National Speleological Society (NSS), Huntsville, Alabama, 463p.
- Lawrence, D.R., & Melson, W.G., 1960, Geology and paleontology of Thorn Mountain Cave: The Baltimore Grotto News, v. 3 (6), p. 95-97.
- Myers, J.O., 1962, Cave physics, in: British Caving: An Introduction to Speleology [second edition] (C. H. D. Cullingford, ed.): Routledge and Kegan Paul, London, p. 226-250.
- Plummer, B., 1962, A note on cave breathing: The Baltimore Grotto News, v. 5 (12), p. 282-287.
- Plummer, W.T., 1969, Infrasonic resonances in natural underground cavities: The Journal of the Acoustical Society of America, v. 46 (5), pt. 1, p. 1074-1080.
- Powers, J., 1981, Confederate niter production: National Speleological Society (NSS) Bulletin, v. 43, p. 94-97.
- Smith, M.O., 1995, The Confederate Nitre Bureau & Virginia’s Nitre District Number Three, in: Underground in the Appalachians (C. Zokaites, ed.): National Speleological Society (NSS), Huntsville, Alabama, p. 187-198.
- Stokowski, Jr., S.J., 1979, Structural, petrographic, and relative solubility relationships in the Sinnett-Thorn Mountain Cave System, Pendleton County, West Virginia: The National Speleological Society (NSS) Bulletin, v. 41 (1), p. 10.
- Swartz, F.M., 1929, The Helderberg Group of parts of West Virginia and Virginia: U. S. Geological Survey Professional Paper 158-C, p. 27-75.
- Swartz, C.K., Schuchert, C., & Prosser, C. S., 1913, Lower Devonian – Text: Maryland Geological Survey, 560p.
- Swezey, C., & Piatak, N., 2003 – in press, Gypsum in Butler Cave, Bath County, Virginia: Butler Cave Conservation Society (BCCS) Newsletter.
- Swezey, C.S., Piatak, N.M., Seal II, R.R., & Wandless, G.A., 2002, The isotopic compositions of sulfur from gypsum in caves of Virginia and West Virginia: Geological Society of America, Abstracts with Programs, v. 34 (6), p. 231. [Reprinted in: Virginia Cellars (2003), v. 8 (1), p. 35]
- Taylor, J.C., 2001, The early history of Pendleton County caves 1760 to 1860, in: The Caves and Karst of Pendleton County (G. Dasher, ed.): West Virginia Speleological Survey Bulletin 15, p. 42-59.
- Ulrich, E.O., 1911, Revision of the Paleozoic System: Geological Society of America Bulletin, v. 22, p. 281-680.
- Wigginton, E., 1979, Foxfire 5: Doubleday, New York, 512p.
- Wigley, T.M.L., 1967, Non-steady flow through a porous medium and cave breathing: Journal of Geophysical Research, v. 72, p. 3199-3205.
- Wigley, T.M.L., & Brown, M.C., 1976, The physics of caves, in: The Science of Speleology (T. D. Ford and C. H. D. Cullingford, eds.): Academic Press, New York, p. 329-358.
Figures







Download Paper
A PDF version of this paper (including figures) is available for download here.